E+E Elektonik launches EE 212 humidity and temperature sensor
ENGERWITZDORF, Austria, 19 May 2021: E+E Elektronik has launched the EE212 humidity and temperature sensor, which it described as being suitable for challenging measurement tasks in climate technology, agriculture and the pharmaceuticals industries.
A modular probe design makes it easy to replace the sensing module directly on site, if needed, E+E Elektronik said, adding that the E+E sensor coating, the wide choice of filter caps, and the robust IP65 / NEMA 4 enclosure ensure accurate and reliable measurements even under challenging working conditions.
Sauermann launches six HVACR measuring instruments
BRUSSELS, Brussels, 07 February 2021: Sauermann’s new range of measuring instruments offers its 40 years of metrological expertise to distributors in the HVACR sector, the company said through a Press release.
Sauermann said it is offering six reliable and precise instruments designed to quickly check the functionality of HVACR systems. The new range of products covers a full spectrum of measurements, including temperature (dual input Si-TT3 and infrared Si-TI3 thermometers), humidity (thermo-hygrometer Si-HH3), pressure (digital differential pressure manometer Si-PM3), and air velocity (hotwire Si-VH3* and vane Si-VV3 thermo-anemometers), it said. Dependable, quick and easy measurements are done with just a few clicks, thanks to the instruments’ long-lasting batteries, it claimed.
The instruments are all equipped with a backlit display and a wireless pairing functionality, to be used with a brand-new mobile application available for iOS and Android devices (Si- HVACR Measurement Mobile App), the company said. Developed in-house at Sauermann, the app offers functionalities, such as the display of additional measurements (calculated parameters), and the recording of measurement campaigns that can be exported as PDF, XML or CSV reports (measurement tables and curves over time), it said. Further, Sauermann said it is offering reliable connected devices, with an integrated magnetic holder for handsfree measurements, with which a wide variety of measurements can be taken with ease and confidence.
ASHRAE releases core recommendations for reducing airborne infectious aerosol exposure
ATLANTA, Georgia, 14 January 2021: The ASHRAE Epidemic Task Force has released new guidance to address control of airborne infectious aerosol exposure and recommendations for communities of faith buildings, ASHRAE said through a Press release.
An infectious aerosol is a suspension in air of fine particles or droplets containing pathogens, such as the SARS-CoV-2 virus, which can cause infections when inhaled, ASHRAE said. They can be produced by breathing, talking, sneezing and other as well as by flushing toilets and by certain medical and dental procedures, it added.
ASHRAE’s Core Recommendations for Reducing Airborne Infectious Aerosol Exposure concisely summarize the main points found in the detailed guidance documents produced by the ASHRAE Epidemic Task Force, it said. They are based on the concept that ventilation, filtration and air cleaners can be combined flexibly to achieve exposure reduction goals, subject to constraints that may include comfort, energy use and costs, it added.
“This guidance outlines a clear approach for lessening the risk of infectious aerosol exposure for building occupants that can be applied in a wide range of applications, from homes to offices, to mobile environments, such as vehicles and ships,” said William Bahnfleth, Chair, ASHRAE Epidemic Task Force. “ASHRAE’s Core Recommendations are based on an equivalent clean air supply approach that allows the effects of filters, air cleaners, and other removal mechanisms to be added together to achieve an exposure reduction target.”
According to ASHRAE, specific recommendations include the following:
- Public health guidance
- Follow all regulatory and statutory requirements and recommendations.
- Ventilation, filtration, air cleaning
- Outdoor airflow rates guidance for ventilation, as specified by applicable codes and standards.
- Recommendations on filters and air cleaners that achieve MERV 13 or better levels of performance.
- The use of air cleaners.
- Control options that provide desired exposure reduction while minimizing associated energy penalties.
- Air distribution.
- Promote the mixing of space air.
- HVAC system operation
- Maintain temperature and humidity design set points.
- Maintain equivalent clean air supply required for design occupancy.
- Operate systems for a time required to achieve three air changes of equivalent clean air supply.
- Limit re-entry of contaminated air.
- System commissioning
- Verify that HVAC systems are functioning as designed.
According to ASHRAE, the task force’s Communities of Faith Buildings guidance offers recommendations on conducting worship services under epidemic conditions.
Rick Karg, ASHRAE Epidemic Task Force member, said: “The intent of the Communities of Faith guidance is to offer those who operate and care for buildings used for worship a plan for implementing short- and long-term HVAC strategies to reduce the possibilities of transmission of the SARS-CoV2-2 virus. The document also helps communities move toward a new ‘normal’ operation after this public health emergency nears an end.”
According to ASHRAE, recommendations for Communities of Faith include the following:
- Identify HVAC system characteristics. Compile and review operation and maintenance manuals and schedules.
- Verify HVAC systems are well maintained and operating as intended. For maintenance, follow the requirements of ASHRAE Standard 180 – 2018, Standard Practice for the Inspection and Maintenance of Commercial HVAC Systems.
- Consider PPE when maintaining HVAC systems, including filters, coils and drain pans.
- Operate HVAC systems, if present, with system fan set to run continuously when building is occupied for services or cleaning.
- Operate the system for a time required to achieve three equivalent air changes of outdoor air (effect of outdoor air, filtration and air cleaners) before the first daily occupancy and between occupied periods, if appropriate. Three equivalent air changes can be calculated using ASHRAE’s Building Readiness Guide.
To view the complete ASHRAE Core Recommendations For Reducing Airborne Infectious Aerosol Exposure and Communities of Faith Building guidance, ASHRAE suggested visiting ashrae.org/COVID-19.
ASHRAE Learning Institute opens registration for Spring online courses
ATLANTA, Georgia, 8 January 2021: ASHRAE Learning Institute announced that registration is open for its 2021 Spring online instructor-led course series. The 16 online offerings, including eight new courses, run from January through June, the Institute said
A new course, ‘Reopening Commercial Buildings: Evaluating Your HVAC System’s Readiness to Mitigate the Spread of SARS-CoV-2’, taking place on January 27, will expound the online ASHRAE COVID-19 details for reopening buildings and the Building Readiness Plan for HVAC systems, the Institute said. The course will help reiterate mitigation strategies available and understand specific buildings arrangements, the Institute added.
The course, ‘Health Impacts of Indoor Air Extraction, Ventilation, and Filtration – Same or Different’, scheduled for February 17, the Institute said, will cover the future design of forced air ventilation systems and the most cost-effective HVAC operational changes and system modifications to improve existing indoor environments in reducing the spread of viruses.
The course, ‘Hospital HVAC – Infection Mitigation, Comfort, Performance’, scheduled for February 23, will address the role of HVAC systems in helping to reduce Hospital Associated Infections (HAI), explaining airborne versus contact transmission, the Institute said. This course will describe the why and how filtration, air patterns, air changes, dilution, temperature, humidity, UV and pressurization in hospital HVAC can either help or hinder efforts to reduce HAI, the Institute added.
According to the Institute, the following is the full schedule of online instructor-led course offerings:
January 26: COVID-19 and Buildings: Re-occupation after Lockdown
January 27: Reopening Commercial Buildings: Evaluating Your HVAC System’s Readiness to Mitigate the Spread of SARS-CoV-2
February 17: Health Impacts of Indoor Air Extraction, Ventilation, and Filtration – Same or Different?
February 23: Hospital HVAC – Infection Mitigation, Comfort, Performance
February 24: Evaluating Your HVAC System’s Readiness to Mitigate the Spread of SARS-CoV-2
March 2: Latest in High-Performance Dedicated Outdoor Air Systems
March 4: Humidity Control I: Design Tips and Traps
March 25: Save 40% by Complying with Standard 90.1-2019
April 6: Commercial Building Energy Audits – Part I
April 13: Commercial Building Energy Audits – Part II
April 20: Air-to-Air Energy Recovery Fundamentals
April 22: V in HVAC – What, Why, Where, How, and How Much
May 4: An Introduction to ASHRAE Existing Building Commissioning
May 11: Fundamentals of Ultraviolet Germicidal Irradiation (UVGI) for Air and Surface Disinfection
May 20: Introduction to BACnet
June 1: Principles of Building Commissioning: ASHRAE Guideline 0 and Standard 202
June 8: Powering with Renewable Resources: Thermal Energy Storage
How to kill enveloped viruses in just 30 minutes
 Poor ventilation in closed indoor environments is associated with increased transmission of respiratory infections. There have been numerous SARS-CoV-2 transmission events associated with closed spaces, including some from pre-symptomatic cases. The role of ventilation in preventing SARS-CoV-2 transmission is not well-defined – that is, by preventing dispersal of infectious particles in small waterdrops to minimise the risk of transmission or preventing transfer of an infectious dose to susceptible individuals.
Poor ventilation in closed indoor environments is associated with increased transmission of respiratory infections. There have been numerous SARS-CoV-2 transmission events associated with closed spaces, including some from pre-symptomatic cases. The role of ventilation in preventing SARS-CoV-2 transmission is not well-defined – that is, by preventing dispersal of infectious particles in small waterdrops to minimise the risk of transmission or preventing transfer of an infectious dose to susceptible individuals.
SARS-CoV-2 is thought to be primarily transmitted through large respiratory droplets; however, an increasing number of outbreak reports implicate the role of aerosols in SARS-CoV-2 outbreaks. Aerosols consist of small droplets and droplet nuclei, which remain in the air for longer than large droplets. Studies indicate that SARS-CoV-2 particles can remain infectious on various materials, such as air conditioning surfaces in air ducts and air handlers, as well as in aerosols in indoor environments, with the duration of infectivity depending on temperature and humidity.
 While HVAC coatings are often the most cost-efficient insurance for the longevity of your air-handling system, there’s much more to them than just increasing your building systems’ lifespan. The rising demand for antimicrobial coatings was triggered by the COVID-19 pandemic and tenants worried about their wellbeing from airborne diseases. In the same category, antimicrobial coatings can make a huge difference for indoor air quality and occupant safety. There are a number of HVAC coatings that drive energy savings, primarily desiccant-coatings.
While HVAC coatings are often the most cost-efficient insurance for the longevity of your air-handling system, there’s much more to them than just increasing your building systems’ lifespan. The rising demand for antimicrobial coatings was triggered by the COVID-19 pandemic and tenants worried about their wellbeing from airborne diseases. In the same category, antimicrobial coatings can make a huge difference for indoor air quality and occupant safety. There are a number of HVAC coatings that drive energy savings, primarily desiccant-coatings.
Found on AHU heat exchangers, coils and in duct systems, they enable recovering heat and moisture, which then helps building owners to save on operational cost. Recent studies have uncovered an extreme antimicrobial effect of desiccant coating systems, in high relative humidity, as present in air conditioning systems. It appears the surfactants can break the exterior protein of a virus or bacteria strain. Once the protein is destroyed, the virus cannot attach to cells and transfer or alter human ribonucleic acid (RNA).
 In many circumstances, once microbes have begun to proliferate on a painted surface, constant cleaning and disinfecting is required to keep growth under control, which is highly unwanted inside an air conditioning system. Recognising that the ability to clean constantly is unreasonable in most air conditioning systems, the best weapon against corrosion and microbial growth is an antimicrobial paint that prevents growth of, or eliminates, bacteria and viruses. Both the coating and the possible active ingredient should not produce any environmental, safety or health issues during application. Any off-gas from the film is unwanted, because ideally, the coating must be applied to air conditioning systems in operation without any concern of release of poisonous additives.
In many circumstances, once microbes have begun to proliferate on a painted surface, constant cleaning and disinfecting is required to keep growth under control, which is highly unwanted inside an air conditioning system. Recognising that the ability to clean constantly is unreasonable in most air conditioning systems, the best weapon against corrosion and microbial growth is an antimicrobial paint that prevents growth of, or eliminates, bacteria and viruses. Both the coating and the possible active ingredient should not produce any environmental, safety or health issues during application. Any off-gas from the film is unwanted, because ideally, the coating must be applied to air conditioning systems in operation without any concern of release of poisonous additives.
Antimicrobial efficacy based on silver ions
Generally, an antimicrobial surface contains an additive, like Agion, which inhibits the antimicrobial property that is composed primarily of silver ions, which have been proven in antimicrobial use throughout history. It incorporates silver ions inside a zeolite carrier, providing an area for these ions to exchange with other positively charged ions – often sodium – from the moisture in the environment.
Once exchanged, these now “free” silver ions are attracted to oppositely charged hydrogen ions, commonly found in most bacteria and microbes. The bacteria and microbes’ respiration and growth are now abruptly halted, since the hydrogen ions are no longer available. Silver based antimicrobial coatings contain a pesticide additive that evaporates slowly from the coating surface and raises questions on the durability of discharge. In Europe and North America, these coatings require a registration by the government authorities.
Antimicrobial efficacy based on desiccation
Enveloped viruses, like the H1N1 influenza virus, Corona (COVID-19) and bacteria have membranes of protein and enzymes to protect the infecting contents. The spreading of the viruses and bacteria in closed spaces and air conditioning systems is carried out by smaller aerosols. Alternative antimicrobial functionality is based on desiccation, a physical process to extract the moisture from the virus and bacteria particles. This approach may seem relatively primitive; however, it is extremely effective in slowing down or even preventing microbes from spreading and transmission. This method is similar to other physical treatments, such as UV irradiation, filtering and heating.
 Desiccant coatings inactivate a wide variety of microbes that adhere to the surface through their hydrophilic surface properties. The antiviral functionality of the coating has been tested on the Phi6 virus, which is commonly used as surrogate for enveloped Corona viruses.
Desiccant coatings inactivate a wide variety of microbes that adhere to the surface through their hydrophilic surface properties. The antiviral functionality of the coating has been tested on the Phi6 virus, which is commonly used as surrogate for enveloped Corona viruses.
Studies
A recent study shows that a desiccant coating can have an extremely quick kill-rate of enveloped viruses after just 30 minutes.
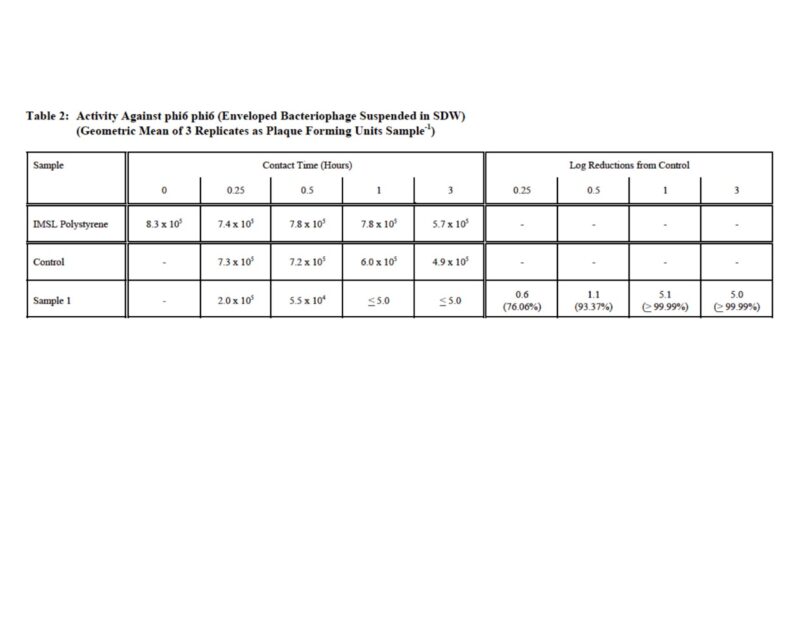 Further studies have proven that strong antimicrobial working was additionally confirmed. Surface activity results in full kill-rates of > 99,99%, which were confirmed on the following micro-organism strains:
Further studies have proven that strong antimicrobial working was additionally confirmed. Surface activity results in full kill-rates of > 99,99%, which were confirmed on the following micro-organism strains:
- Salmonella
- Legionella
- E-Coli
- MRSA
- Klebsiella Pneumoniae
An important note should be added to this paper: No claim or assertion should be made that the antimicrobial properties in the coating will improve air quality or eliminate the threat of disease-causing microbes in the air supply system. A healthy indoor air system is highly dependent on a combination of design, maintenance and cleaning measurements that are incorporated in the air conditioning system and facility management procedures.
- Knibbs LD, Morawska L, Bell SC, Grzybowski P. Room ventilation and the risk of airborne infection transmission in 3 health care settings within a large teaching hospital. Am J Infect Control. 2011 Dec;39(10):866-72.
- Lu J, Gu J, Li K, Xu C, Su W, Lai Z, et al. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020 Apr 2;26(7).
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020 Mar 5;382(10):970-1.
- World Health Organization (WHO). Natural Ventilation for Infection Control in Health-Care Settings. 2009 [updated 4 May 2020].
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323(16):1610-2.
- Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR. Airborne or droplet precautions for health workers treating COVID-19? The Journal of Infectious Diseases. 2020.
- Dietz L, Horve PF, Coil DA, Fretz M, Eisen JA, Van Den Wymelenberg K. 2019 Novel Coronavirus (COVID19) Pandemic: Built Environment Considerations To Reduce Transmission. mSystems. 2020 Apr 7;5(2):e00245-20.
8 Evaluation of Phi6 Persistence and Suitability as an Enveloped Virus Surrogate Aquino de Carvalho, Nathalia; Stachler, Elyse N.; Cimabue, Nicole; Bibby, Kyle Environmental Science & Technology (2017), 51 (15), 8692-8700CODEN: ESTHAG; ISSN:0013-936X. (American Chemical Society)
Recent outbreaks involving enveloped viruses, such as Ebola virus and SARS COVID-2, have raised questions regarding the persistence of enveloped viruses in the water environment. Efforts have been made to find enveloped virus surrogates due to
challenges investigating viruses that require biosafety-level 3 or 4 handling. In this study, the enveloped bacteriophage Phi6 was evaluated as a surrogate for enveloped waterborne viruses. The persistence of Phi6 was tested in aq. conditions chosen based on previously published viral persistence studies. Our results demonstrated that the predicted T90 (time for 90% inactivation) of Phi6 under the 12 evaluated conditions varied from 24 minutes to 117 days depending on temperature, biological activity, and aq. media compn. Phi6 persistence was then compared with persistence values from other enveloped viruses reported in the literature. The apparent suitability of Phi6 as an enveloped virus surrogate was dependent on the temperature and compn. of the media tested. Of evaluated viruses, 33%, including all conditions considered, had T90 values greater than the 95% confidence interval for Phi6. Ultimately, these results highlight the variability of enveloped virus persistence in the environment and the value of working with the virus of interest for environmental persistence studies.
- The use of bacteriophages of the family Cystoviridae as surrogates for H5N1 highly pathogenic avian influenza viruses in persistence and inactivation studies
Adcock, Noreen J.; Rice, Eugene W.; Sivaganesan, Mano; Brown, Justin D.; Stallknecht, David E.; Swayne, David E.
Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances & Environmental Engineering (2009), 44 (13), 1362-1366CODEN: JATEF9; ISSN:1093-4529. (Taylor & Francis, Inc.)
Two bacteriophages, .vphi.6 and .vphi.8, were investigated as potential surrogates for H5N1 highly pathogenic avian influenza virus in persistence and chlorine inactivation studies in water. In the persistence studies, .vphi.6 and .vphi.8 remained infectious at least as long as the H5N1 viruses at both 17 and 28 degrees C in fresh water, but results varied in salinated water. The bacteriophage .vphi.6 also exhibited a slightly higher chlorine resistance than that of the H5N1 viruses. Based upon these findings, the bacteriophages may have potential for use as surrogates in persistence and inactivation studies in fresh water.
- Systematic Review and Meta-Analysis of the Persistence and Disinfection of Human Coronaviruses and Their Viral Surrogates in Water and Wastewater, Andrea I. Silverman and Alexandria B. Boehm, April 2020
- Determination of the Antiviral Activity of Water-Based Coating for Air Conditioning Applications against phi6 Bacteriophage using a Method Based on ISO 21702:2019, the laboratories of Industrial Microbiological Services Ltd at Pale Lane Hartley Wintney, Hants, RG27 8DH, UK. December 2020
The writer is with Aqua Aero Coatings and may be contacted at wouter@aquaaero.net
UPS delivers Pfizer vaccine in Saudi Arabia

RIYADH, Saudi Arabia, 21 December 2020: UPS said it has successfully delivered the first batches of the Pfizer-BioNTech COVID-19 vaccine in Saudi Arabia, to support vaccinations of first citizens and expatriates. Making the announcement through a Press release, UPS said Saudi Arabia is the first Arab country to roll out the Pfizer-BioNTech jab, marking a breakthrough milestone in the ongoing response to the COVID-19 pandemic.
Rachid Fergati, UPS Managing Director for Middle East and the Indian subcontinent, said: “UPS has proudly delivered the first batches of the Pfizer-BioNTech COVID-19 vaccine to Saudi Arabia, to support vaccinations of first citizens and expatriates in the country.
“Saudi Arabia is the first country in the Middle East that we are serving, and we are in position to continue delivering what matters to help stamp out the pandemic in the region.
“We have spent months developing new products, agile approaches and new capabilities to ensure we are fully prepared to deliver the vaccine at the right time, at the right temperature to communities all over the world, especially here in the region.
We are honored to work with UPS Healthcare partners in other countries to help deliver what matters in these times.”

‘Clear-cut instructions needed to address cold chain breaches in the healthcare industry’
What do you consider to be the main issue plaguing the modern medical cold chain, with regard to avoiding temperature excursions that could potentially degrade the potency of medication and vaccines?
The main issue is the lack of international or uniform guidelines to instruct health providers/ consumers about “what to do in case of a cold chain breach”? We do know very well how those drugs should be kept, but we do not know how to manage if a cold chain breach (CCB) occurs. There is a clear lack of guidelines for these possible incidences. These incidences are not just limited to developing countries, but they could happen anywhere at any time; an example is a power outage due to maintenance/upgrade or even natural disasters that cannot be anticipated.
Does it vary from developed and developing countries as the level of investment among them must vastly differ? Do you see lack of innovation in equipment as a problem or is the problem mostly adoption and investment in more reliable equipment? Similarly, is there a gap in further training and awareness in the ‘last mile’?
Developed countries usually have a more reliable infrastructure and power supply, as compared to developing countries. But when you think of the level of investment in these countries, often developed countries rely on their reliable infrastructure and may not see the need to invest further on this. Evidence of this is our recent research that showed the lack of guidelines and concerns about awareness and planning in Australia, which is a developed country. Whereas developing countries are aware of their infrastructure limitations but may have difficulty in investing.
Certainly, there is an evident gap in training and awareness. I believe developing user-friendly guidelines that are evidence-based, accurate and easy to understand, would significantly help. For example, imagine if an expensive biological drug that is worth about USD 1,000 and is stored in a pharmacy or a hospital fridge is exposed to a higher temperature, due to a temporary power outage for certain number of hours. Although the temperature records are available, there is no reliable instruction to healthcare providers whether the drug is usable anymore or not? So individuals need to use their judgement whether to expose the drugs (that could be quite costly) or to continue supplying it to their patients (that could bear a risk). Developing guidelines can provide clear-cut instructions on how to deal with those situations. Data from a small local study indicate millions of dollars worth of medicines could be saved each year if additional data regarding the stability of medicines were available to the relevant parties
Could you briefly comment on the impact these power outages could have on the integrity of the vaccines and medicines?
To explain the impact of power outage and CCB, we can look at the global burden, as identified by the WHO. The WHO estimates that up to 50% of vaccines may be wasted globally every year because of temperature control, logistics and shipment-related issues. We found the gap in the knowledge, as we did not find any comprehensive international study that assessed the impact of CCB on non-vaccine medicines (those that need to be refrigerated). In our small local study we found that power outages could lead to significant financial loss, either to the healthcare providers that store the drugs or to the insurance companies, with regard to vaccination. The worst thing that could happen is that if healthcare providers or suppliers overlook a potential CCB and the medicine reaches the consumer, usually there is no way to assure the integrity of the medicines by the consumers or even by the healthcare providers. That may result in under-vaccination, without knowing about the potential CCB, and subsequently could predispose people to communicable diseases.
Are the government guidelines enough, in terms of securing a stringent cold chain? Where do you think are the most prominent gaps and what are your recommendations?
No. Government guidelines are often quite broad and do not provide practical solutions once CCB has occurred. For example, the WHO has developed a set of guidelines for governments in a bid to minimise exposure to high temperatures, if a power outage happens. But these guidelines don’t have any specific instructions on how healthcare facilities and pharmacies should implement backup systems. They also don’t provide a list of standardised equipment to prevent and deal with power outages. This would be helpful in both developed and developing country scenarios.
The most prominent gap is the lack of uniform evidence-based guidelines about transportation and storage or vaccines and medicines. Given that most pharmaceutical companies these days are international, similar or identical medicines are being marketed in different countries, so if manufacturers conduct comprehensive stability testing and transparently provide the information to the public, by collaboration between independent scientists and manufacturers, we can develop these guidelines.
Should energy insecure countries be more vigilant?
Yes, where power supply is not reliable, there is an increased incidence of power outage that can affect the integrity of medications. An interesting example is, when there was an Ebola outbreak in 2014 and or after the Nepal earthquake in 2015, there was not a reliable power source, due to being in remote locations and the natural disaster that affected the infrastructure, respectively. A vaccine storage device was developed and trialled that was called Arktek. It is a super-insulated device that maintains the integrity of vaccines by keeping them in ice, in its inner chamber. It can keep vaccines at a temperature between zero degrees C and eight degrees C for 30 to 60 days, depending on outside temperatures and humidity. Although this might have worked well in those outbreaks in remote areas, however, it may not be possible to use these devices in all settings.
You mentioned that actual cost of vaccine and pharmaceutical loss is poorly studied and requires further research. Could you comment on what are the key areas that you believe should be given more attention?
The key areas include vaccines that are extremely important to the public health and biological drugs that are quite costly for the consumers/health insurance/public healthcare systems.
Manufacturers can invest on developing heat-stable drugs so that drugs can be stored outside the cold chain for a longer time. This will significantly contribute to preventing wastage in medications and saving in logistics. But we should acknowledge that, this may not be so simple.
How can manufacturers help address the issue?
Manufacturers could conduct more vigorous stability testing, share their data transparently with independent scientists and invest in developing guidelines to deal with power outages for their own products. This is the least you can expect from manufacturers, especially for biological drugs, where usually each supply of the drug that lasts for a month is worth at least USD 1,000 or even more. So the investment is well justified.


